Abstract
Background:
FLT3 ITD AML is associated with high rates of relapse and short relapse free survival. Prognosis after relapse is extremely poor, making optimization of initial therapy for this group of patients important. Most data now suggest allogeneic transplant should be the standard consolidation for FLT3 ITD AML. Although peri-transplant tyrosine kinase inhibitor (TKI) use is common, there is no consensus on the best way to incorporate TKIs into the management of these patients. A comprehensive review of treatment outcomes is needed to determine which strategies reduce the risk of relapse and prolong survival.
Methods:
We conducted a retrospective chart review for 112 patients with FLT3 ITD mutations seen at JHH between 2005 and 2016 who underwent bone marrow transplantation to collect demographic, diagnostic, transplant and survival data. Differences in the distribution of demographic, clinical, and biological characteristics were analyzed for significance using Fisher's exact test. Consolidation was defined as any cytarabine-based regimen after remission induction therapy. MRD positivity included the presence of >0 to 5% abnormal blasts by morphology or flow cytometry and/or persistence of genetic abnormalities (cytogenetics, mutation panels) prior to transplant. Overall survival (OS) was defined as the time interval from date of transplant to date of death from any cause or to last follow up visit. Relapse free survival (RFS) was defined as the time interval from date of transplant to date of relapse or death from any cause. Survival was censored at 2 years post-transplant. Time was censored at the last follow-up visit if relapse or death were not observed. Survival estimates were calculated using Kaplan-Meier method and comparison of survival distributions were done using log-rank test. TKI included are quizartinib, sorafenib, and gilteritinib.
Results:
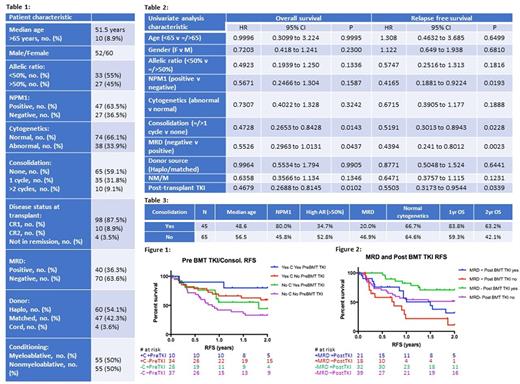
Patient characteristics are summarized in Table 1.
Outcomes based on disease characteristics including NPM1 status, allelic ratio and cytogenetics are listed in Table 2. Patients with NPM1 mutations had improved RFS (HR=0.4165, p=0.0193). Patients with no detectable MRD prior to transplant have improved RFS (HR=0.4394, p=0.0023) and OS (HR=0.5526, p=0.0437).
Outcomes related to treatment strategies are also summarized in Table 2. Patients with at least 1 cycle of post remission consolidation had lower rates of MRD (p=0.0046) (Table 3), and improved RFS (HR=0.5191, p=0.0228) and OS (HR=0.4728, p=0.0143) (Figure 2). Patients who received consolidation and pre-transplant TKI had the best outcomes (Figure 1). In the absence of consolidation, pre-transplant TKI usage was associated with longer RFS (median 1.819 years versus 0.8548 years, p = 0.2105) and OS (median 1.995 years versus 1.249 years, p=0.4384) compared to those with no pre-transplant TKI use. Patients who received either consolidation or pre-transplant TKI had similar outcomes. Non-myeloablative conditioning regimens trended toward favorable outcomes over myeloablative regimens despite older patients and lower rates of NPM1 mutations (HR=.6358, p=0.1346 for OS, HR=0.6471, p=0.1231 for RFS). There was no difference in OS or RFS between matched and haploidentical donors. Post-transplant TKI use improves RFS (HR=0.5503, p=0.0339) and OS for all patients (HR=0.4679, p=0.0102). For patients who had MRD prior to transplant, TKI use post-transplant was associated with median RFS of 1.499 years versus 0.7425 years for those who did not use post-transplant TKI (Figure 2). Post-transplant TKI use also improved OS (HR=0.3737, p =0.0308) for patients without MRD prior to transplant.
Discussion:
Remission status at time of transplant is key to outcomes. Consolidation, as well as use of TKI pre- and post-transplant are associated with favorable outcomes. Although patients who were given consolidation had more favorable outcomes, it is unclear if patient characteristics (higher NPM1 %, lower AR>50%, and younger age) confounded their outcomes. Matched and haploidentical donors confer similar outcomes. This data supports treatment of FLT3 ITD AML patients to include induction, 1 cycle of post remission consolidation, TKI use prior to transplant, and transplant with post-transplant TKI maintenance.
Smith: Celgene: Consultancy; Curis: Consultancy; Ariad/Takeda: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Swinnen: Genentech: Consultancy; Pharmacyclics: Consultancy. Levis: Astellas Pharma Us: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; FujiFilm: Research Funding; Daiichi Sankyo, Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal